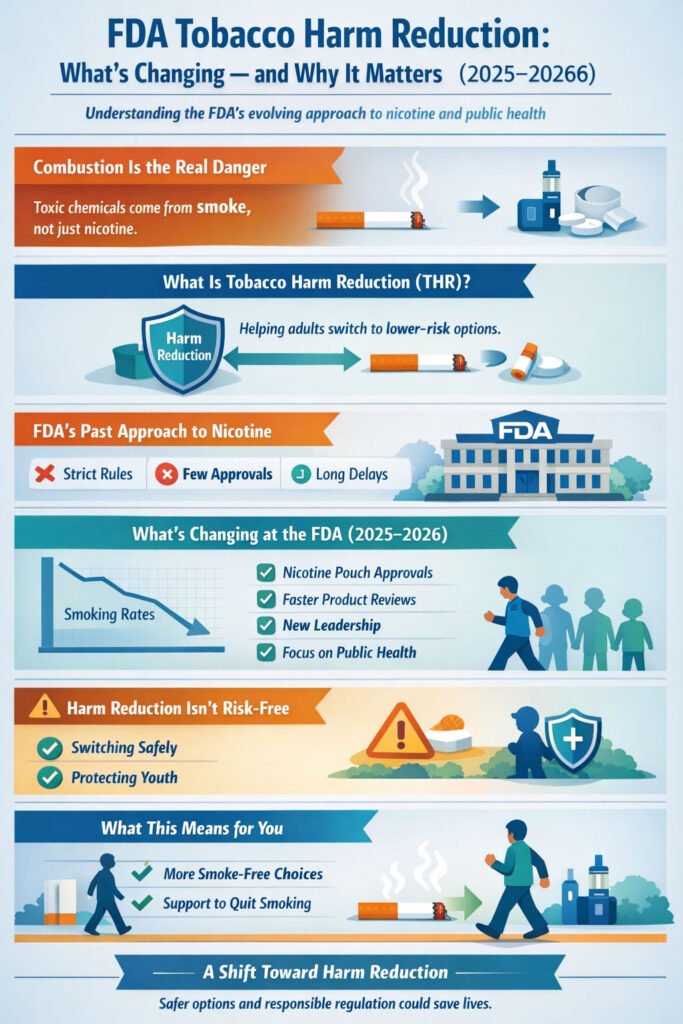
In 2025 and 2026, the U.S. Food and Drug Administration (FDA) appears to be shifting its stance on how it regulates nicotine and tobacco products — potentially signaling a thaw toward tobacco harm reduction (THR). This change could have implications for adults who smoke, public health policy, and how safer alternatives are evaluated and made available in the United States.
While the FDA has historically taken a cautious and often restrictive approach, recent developments suggest that the agency may be moving toward a broader recognition of harm reduction strategies — including recognizing that some nicotine products are much less harmful than combustible cigarettes and can play a constructive role in reducing smoking-related disease. Filter+1
What Is Tobacco Harm Reduction?
Tobacco harm reduction is a public health approach focused on reducing the health risks associated with smoking by encouraging adults who smoke to switch completely to lower-risk nicotine products. These alternatives include:
- Nicotine pouches
- E-cigarettes (vapes)
- Heated tobacco products
- Other non-combustible nicotine delivery systems
Unlike cigarettes, these products do not burn tobacco — the process responsible for the vast majority of smoking-related disease and death. By separating nicotine from the harmful smoke, THR aims to protect health while acknowledging that nicotine addiction itself is not the primary cause of the worst health outcomes. ccagw.org
Recent Signals of FDA Shifts
1. Broader Authorization of Lower-Risk Products
In 2025, the FDA authorized several nicotine pouch products, acknowledging that they could offer greater benefits to public health compared with cigarettes. This is notable because it marks a rare recognition within FDA regulatory processes that certain smoke-free products may help reduce harm. Filter
The agency also initiated pilot programs to streamline the review process for nicotine alternatives. The goal is to focus reviews on the most essential scientific information and shorten the time it takes for certain products to be evaluated and authorized. Filter
2. Leadership Changes and New Perspectives
There have been changes in leadership at the FDA’s Center for Tobacco Products (CTP), which oversees the regulation of tobacco and nicotine products. Health policy observers note that this turnover could be contributing to a different regulatory tone and openness to reforms that previously faced resistance. CSNews
3. Flavored Vape Authorization and Future Possibilities
The acting head of the FDA’s tobacco office has publicly stated that expanded authorization of vaping products — including potentially flavored options — could be on the horizon. This is a stark contrast to the past decade, when very few vaping products received market authorization and restrictions on flavors were a central focus of policy debates. Vaping360
Why This Matters for Public Health
Reducing Smoking Rates
One of the key potential benefits of embracing harm reduction is accelerated declines in smoking prevalence. Scientific research — including FDA’s own modeling — has shown that lowering nicotine in combustible cigarettes to minimally or non-addictive levels could dramatically reduce smoking rates and save millions of lives over the coming decades. U.S. Food and Drug Administration+1
By reducing nicotine dependence, more people may find it easier to quit smoking entirely or to switch to products associated with far lower levels of toxic chemical exposure. U.S. Food and Drug Administration
The Importance of Complete Switching
It is critical that people who smoke completely switch from cigarettes to a lower-risk alternative rather than merely using both products. Partial switching can mitigate some harms, but the most meaningful health benefits occur when cigarettes are fully replaced. U.S. Food and Drug Administration
The relative risk reduction from such switching is significant. While no tobacco-related product is safe, using smoke-free alternatives typically exposes users to far fewer harmful chemicals than smoking. U.S. Food and Drug Administration
Where the Debate Still Stands
Despite these developments, the regulatory landscape remains complex and contested:
Critics and Concerns
Public health advocates worried about youth uptake and long-term nicotine addiction have urged caution. They argue that expanded access to flavored or popular harm reduction products could unintentionally attract non-smokers, particularly youth, to nicotine use.
There is also ongoing tension between strict tobacco control policies — such as flavor bans and menthol cigarette restrictions — and harm reduction advocates who believe those bans may push adult smokers back to cigarettes rather than toward safer options. Filter
Regulatory and Legal Realities
Even as the FDA considers harm reduction pathways, some aspects of tobacco regulation remain in flux. For example, proposed rules aimed at reducing menthol cigarettes have faced delays and pushback, illustrating that change is not uniform across all nicotine policy areas. Filter
What Advocates Say
Supporters of tobacco harm reduction argue that the FDA should:
- Authorize a wider variety of smoke-free nicotine products
- Shorten and simplify review processes so safer products can reach the market faster
- Provide clearer communication to the public about relative risks
- Educate health professionals about harm reduction strategies
They point to countries like Sweden, where widespread use of oral nicotine products has been linked to some of the lowest smoking rates in Europe, as a model for how policy can encourage harm reduction without increasing youth nicotine use. Filter
What This Means for People Who Smoke
If the FDA continues on this path, it could result in:
More Approved Harm Reduction Options
A broader regulatory embrace could make more alternatives to cigarettes legally available in the U.S.
Better Support for Switching
Improved communication and education could help adults who smoke understand how to transition safely to lower-risk products.
Faster Innovation
A more flexible review process could reduce delays that previously impeded the availability of safer nicotine products that adult smokers might use to quit cigarettes.
A Balanced Approach Still Needed
While harm reduction strategies show promise, they should be part of a comprehensive public health framework that includes:
- Robust smoking cessation support and treatment
- Clear risk communication
- Youth prevention efforts
- Monitoring of product use trends
Reducing smoking’s harm doesn’t mean abandoning public health goals — it means recognizing that adults who already smoke need realistic, science-based pathways to healthier alternatives. And as regulatory attitudes shift, the potential to accelerate smoking declines in the U.S. may grow. ccagw.org
Conclusion: A New Chapter in Tobacco Regulation?
The FDA appears to be at a possible turning point in how it approaches tobacco and nicotine regulation. After years of strict controls and limited product authorizations, recent signals suggest increased openness to harm reduction pathways that could benefit millions of adults who smoke. While progress is incremental and debate continues, 2025–2026 may emerge as a period of important change and opportunity.
For policymakers, health professionals, and people who smoke, staying informed about these shifts could help guide healthier decisions and support efforts to reduce tobacco-related disease in the years ahead.